April 2022, Vol. 249, No. 4
Features
Engineer’s Role in Hazardous Area Classification
By Chandragupthan Bahubali, Saravanan Murugesan and Eswaran Marimuthu, Wood Plc., India
Hazardous area classification is not a new technique to address the risk associated with flammable gas, vapor, liquids and dust. It involves evaluating a location or process for hazards of fire or the probability of incident.
In the process of hazardous area classification, the plot plan is divided into areas by risk levels. Each area is classified based on the ventilation, and risk is assessed based on the gas group and zones to finalize the electrical installation requirements.
Elements of hazardous area classification are types of hazards present, such as gases, vapors, liquids and dusts, along with possibilities of potentially explosive conditions present.
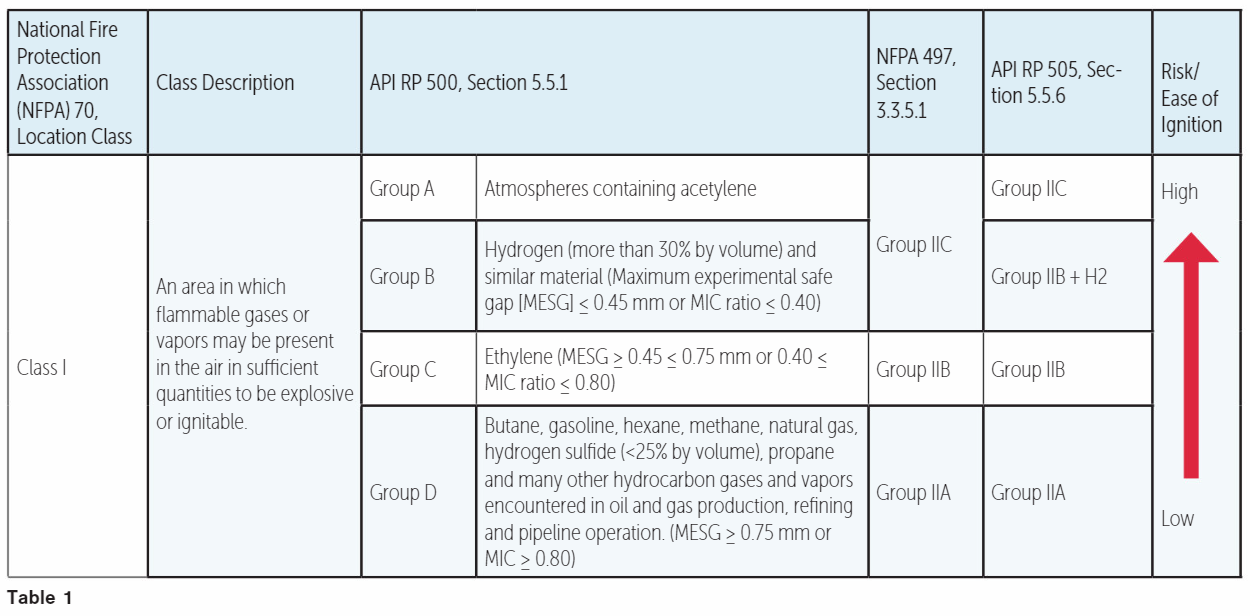
Flammable gas and vapors are grouped into four classes (Table 1), based on the minimum ignition energy (MIC).
Flammable/Combustible Liquids: NFPA 30, Section 4.3, classifies the flammable and combustible liquids based on their flashpoint, since the flashpoint indicates the lowest temperature in the presence of air at atmospheric pressure, at which a flammable liquid will form an explosive/flammable mixture.
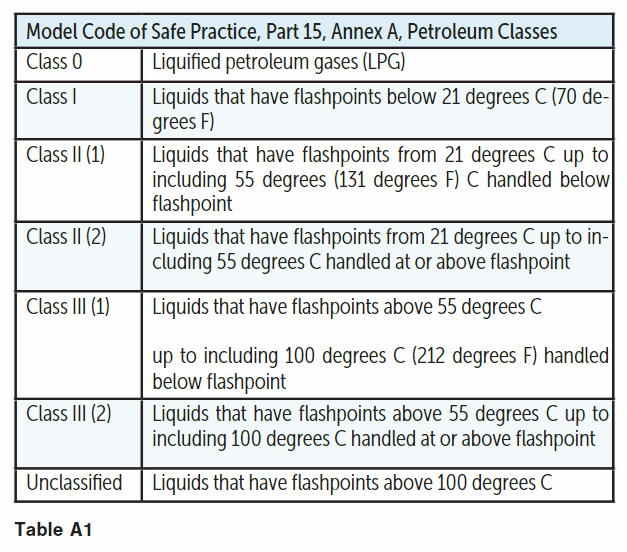
Model code of safe practice, Part 15, accounts for operating temperature as well in its classification. It acknowledges that the release may or may not form mist/spray and it depends on temperature, pressure, hole size, etc. When the fluid temperature is 5 degrees C below its flash point and at atmospheric pressure or under only a few meters head in a storage tank, it can be treated as non-hazardous.
Model code of safe practice, Part 15, Annex A, Table A1 classifies petroleum based on its flash point and operating temperature as detailed in the table.
Cryogenic Flammable Liquids: As defined in NFPA 497, Section 4.2.5, cryogenic liquids are generally handled below -150 degrees F (-101 degrees C). Small liquid spills will immediately vaporize, but larger spills will remain in a liquid state for an extended time and may form an explosive vapor cloud. The dispersion behavior will be based on many factors such as its density, ventilation, wind velocity, ambient conditions, etc. NFPA 59A can be used for liquefied natural gas (LNG) services.
Biofuels: Limited experimental data are available for biofuels. Recent studies show that a mixture of ethanol and gasoline has lower autoignition temperature because of the promotion of isooctane combustion by ethanol.
High Volatile Liquid (HVL): API RP 500, Sections 5.3.1, 5.3.2 and API RP 505, Section 5.3 outlines the demarcation of high volatile liquids (HVL). It includes liquids such as butane, ethane, ethylene, propane, propylene, liquified natural gas, natural gas liquids and similar mixtures and vapor pressures that exceed 276 kilopascals (40 psia) at 37 degrees C (100 degrees |F).
It acknowledges the hazard associated with the accidental release of HVL to the atmosphere, which could result in the formation of a large volume of vapors/gases whose density exceeds that of air. Process engineer must analyze the possible location of release and ventilation. If the source is from an elevated location, dilution due to ventilation may be considered, but case-to-case analysis is required. Process engineer must address the presence of HVL in the document.
Mists/Aerosols: Mist is formed due to pressurized sprays, condensation aerosols, agitations/splashing and air stripping. Mist produced by sprays may have larger droplets than that produced by condensation aerosols. Generally, the larger the drop diameter, the lower the flammability limit. Mist consists of small droplet sizes (<20 μm), and the LEL is approximately equal to vapor mixture.
Latest research reports show that the LEL of a mist can fall to as low as 10% of the LEL of the vapor of the same substance. Literature indicates that the mists are difficult to ignite since the MIE and MESG are all slightly higher than their vapor.
Research conducted on heat-transfer fluid (HTF) has concluded that HTF with higher density will form smaller droplets upon leaking. The HFT with higher viscosity is less likely to form an aerosol. The HTF with the higher surface tension will foam larger droplets on leaking.
Higher operating pressure will produce aerosols closer to the leak and smaller mean droplet diameters. It is important that a significant quantity of aerosols may form from HTF at conditions well below their flashpoints.
API RP 500, Section D.8.1 details mist classification and cautions that the strict application of area classification for gases and vapors may not be appropriate because the flammability characteristics of mists are not always predictable. 29 CFR 1910:1200 provides criteria for flammable aerosols, but it is difficult to classify the temperature class/gas group based on this classification. Based on the minimum ignition distance test, results can help to classify the gas groups.
Aerosols may contain flammable gas, liquids and solids. Currently, mist can be considered as equivalent vapor atmosphere in terms of gas group and temperature class. However, in a multicomponent system, which contains light hydrocarbons, the analysis becomes more complex since the lighter fractions evaporate preferentially in a spray, resulting in airborne droplets with different compositions to that of liquid in the pressurized vessel. In such a scenario, environmental aspect must be considered.
Prolonged contact of mist with a hot surface may result in the reforming/cracking of the hydrocarbon and the release of reactive vapors. The relationship between droplet size and lower flammability limit (LFL) is more complex to understand, but LFL is reduced with higher droplet sizes, since the vertically downward motion of the droplet and upward motion of the flame front improves the contact between them.
Various theories have been put forward to explain the mechanism with the support of experiment/field experience, producing inconclusive results; however, industry accepts classification based on equivalent vapor atmosphere in the absence of guidelines, codes and standards.
Frequency of Exposure
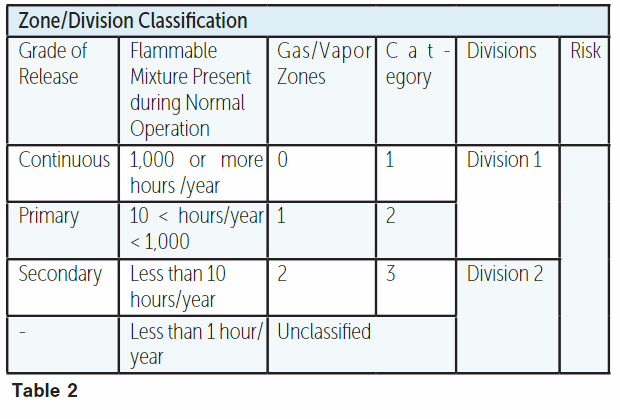
The grade of release is solely dependent on the frequency and duration of release. It is independent of the rate and quantity of the release, degree of ventilation and fluid properties, although these factors contribute to the extent of hazards. The relationship between the frequency or probability of leak and classification of zone/division is shown in Table 2.
Ease of Ignition
Autoignition temperature (AIT) is a function of the concentration of vapor, volume of vapor, pressure of the system, presence of catalytic material, fluid motion (turbulence) and flow conditions. Composition affects AIT; rich or lean mixtures have higher AITs. Larger system volume decreases the AIT because the larger volume has a smaller surface area (volume /surface ratio is high), which results in poor heat transfer and lower AIT.
An increase in pressure (gas concentration is a function of pressure) decreases the AIT since high pressure favors the higher reaction rate, which subsidizes the higher heat transfer (loss). An increase in oxygen concentration decreases the AITs.
During the assessment of the hazardous area classification, normally engineers ignore the circumstances, but the AIT is strongly dependent on the circumstances. It is difficult to estimate the AIT for multicomponent mixtures considering the above factors. As a conservative approach, the component with more than 2% by volume should be considered for analysis.
The addition of components with a lower AIT to a mixture decreases the AIT of the mixture. It can be approximated/inaccurately predicted that the mixture AIT is the AIT of the component with the lowest AIT.
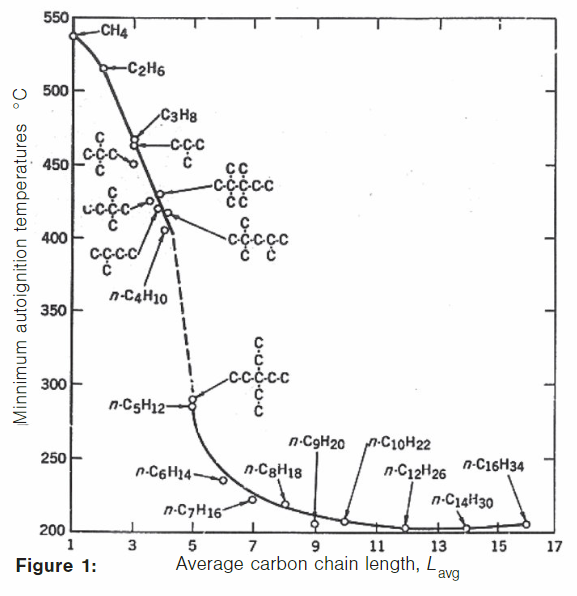
Zabetakis et al. discovered that AIT of hydrocarbon/air mixture decreases with increasing molecular weight and increasing chain length as shown in Figure 1.
AIT is also higher for branched-chain hydrocarbons than straight-chain hydrocarbons since the activation energy required to distract a CH3 is higher for branched-chain hydrocarbons. However, experiments conducted on dotriacontane (C32H66) indicate that the AIT is somewhere above 300 degrees C. Note that the curve turns upward after carbon number 16. In conclusion, AIT is not a constant for any given mixture.
Minimum Ignition Energy (MIE): The minimum ignition energy of gas/vapor decreases with an increase in pressure. An increase in nitrogen concentration increases the MIE.
Flammability Limits: Limits of flammability are affected by various factors, including temperature, pressure, oxygen concentration, inert concentration, size of equipment, direction of flame propagation, turbulence, gravitational field strength, etc. If the flammability limit is not known, ISO10156 can be used, which provides calculation methodology to determine whether the mixture is flammable or not. It does not determine the flammability range.
This standard can be used for determining whether a gas is flammable in air and whether a gas is more or less oxidizing than air. Pressure had little effect on LFL except at extremely low pressures (<50 mm Hg absolute) where flames do not propagate. The upper flammability limit increases slightly as pressure is increased, broadening the flammability range.
Flammability limit changes with the presence of inerts, temperature and pressure. In the absence of flammability data, Le Chatelier’s rule can be used for careful estimation. Many empirical correlations are available, which predict the flammability limits of the mixture and relate the relation between boiling point, flashpoint and flammability limit, etc. But the application requires sound engineering judgment.
When the oxygen concentration is above 21% v/v, it tends to enhance combustion and oxygen level below 21% v/v, causing a decrease in ignition sensitivity and non-flammable conditions at the limiting oxygen concentration value, usually in the range of 9% to 15% by volume.
Ventilation
According to API 500/505/NFPA 497, Section 3.3.1, a ventilation rate that affords six air changes per hour, 1 cubic foot per minute per square foot of floor area (0.3 m3/minute/m2) or other similar criteria prevents the accumulation of significant quantities of vapor and air concentrations from exceeding 25% of the lower flammable limit.
The average wind velocity is high enough to dilute the gas/vapor leak, which may exist within the plant. In the absence of a wall, enclosures, confined space, and air currents, vapor/gas dispersion will depend on density and velocity.
Denser gas/vapor will disperse downward and outward; lighter gases will move upward and outward. Vapor/gas released (high-density release) at or near ground level will be found below ground, altering the shape of a hazardous area.
A mild breeze may extend the hazardous area and a strong wind could dilute the flammable concentration, making it nonhazardous. Normally, mixtures contain components that are both heavier and lighter than air. As a good engineering practice, 80% of the air density at standard condition is considered to classify the heavy and light vapors/gases.
If the vapor/gas is heavier than 80% density of air, then it will be considered as heavier than air. Some process system exhibits poor dispersion quality/lower risk upon release due to high density or viscosity (heavy oils, viscous fluids), high pressure and low volume (chemical injection system), high inert content (high CO2, N2 streams) and high water cut streams.
Electrical equipment must be operated in such a condition that it does not become a source of combustion. This can be prevented by not allowing accidental exposure of sparks or surface temperature of equipment given beyond ignition.
Equipment is rated according to temperature classes based on the maximum temperature that any of its surfaces will reach with reference to an ambient temperature of 40 degrees C. Temperature class with respect to maximum surface temperature details can be referred to in NFPA 70/EI 15 Part 1. Equipment of an appropriate temperature class must be selected based on its operating environment to ensure that any hot surfaces will not ignite the explosive atmosphere.
For example, an atmosphere in which propane may be present, which has an AIT of 450 degrees C has a temperature class of T2; hench, it requires equipment rated to classes T2 or higher (T3). AIP 505 does not address maximum allowable surface temperature requirements. Temperature class with respect to maximum surface temperature details can be referred to in NFPA 70.
Limitations
Hazardous area classification drawings should not be used to determine the equipment layout but may be considered. It does not cover the risk associated with catastrophic failure. It focuses only on electrical apparatus/equipment, and other sources of ignition have been ignored.
Hazardous area classification cannot be used where there are unavoidable ignition sources present, such as gas turbines and fired heaters. Hazardous area classification will not address the consequences of the leak.
Currently available codes and standards do not adequately address the potential explosion risk associated with the vaporization of liquids, mists/spray/aerosol, hybrid mixtures, powders and nanomaterials.
Additionally, explosion and ignition properties tabulated in NFPA 497, Table 4.4.2, cover a limited number of chemicals and components, which is not the real scenario when plants handle mixtures. None of these codes and standards address flammable atmospheres outside normal atmospheric conditions or where the oxidant is not air.
Codes do not classify the locations of explosion hazards due to the presence of high explosives, such as dynamite, TNT or fuel oil mixtures.
Authors:
Chandragupthan Bahubali works as principal process engineer in Wood PLC India (previously Amec Foster Wheeler). He has more than 15 years of post-graduate experience in oil and gas projects. He holds a master’s degree in refining & petrochemical engineering from the University of Petroleum & Energy Studies and a bachelor’s degree in chemical engineering from Madras University, India.
Saravanan Murugesan is a senior process engineer at Wood PLC India. He has more than 10 years of design experience in oil and gas projects. He holds a bachelor’s degree in chemical engineering from Anna University, Chennai.
Eswaran Marimuthu is a process engineer at Wood PLC India. He has more than seven years of design experience in oil and gas projects. He holds a bachelor’s degree in chemical engineering from Anna University, Chennai.





Comments