July 2012, Vol. 239 No. 7
Features
Knowing The Ins And Outs Of pH Sensors
This article talks about 1) knowing when to do a pH sensor calibration versus a calibration check, 2) properly cleaning a pH sensor and 3) properly calibrating a pH sensor. It also provides a decision tree for step-by-step guidance.
Cleanliness looms large in ph sensor care. All pH readings are supposed to be taken and accepted only when the pH sensor is clean. After all, a contaminated pH sensor may yield an incorrect reading. So one must make sure the sensor is clean before doing a calibration. Once a pH sensor is installed in the process and operating, how does one determine when it is time to take the sensor out of the process and do a cleaning or a calibration? Does one perform both a cleaning and a calibration or just a cleaning, or just a calibration, or does one just perform a calibration check in buffers or…?
This is something that can be quite confusing, especially when the operational practices and procedures documented by one’s company’s Quality Control or Environmental Practices department may not be specific enough when they describe the procedure or the timing on when to conduct the pH calibration and maintenance. Conversely, the procedures may be too specific, detailing many more procedures and operations than are actually required.
In practical terms, users must develop their own maintenance and calibration schedule. This schedule is accomplished by taking the pH sensor out of the process after a set amount of time, perhaps after a day or two to perform a visual inspection of the sensor. If – after inspection – you find no debris or fouling on the electrode and reference surfaces with the naked eye, rinse the sensor off in distilled water and perform a buffer check.
To perform a buffer check, place the sensor into the calibration buffers you typically use and note the readings. If the readings are within the tolerances defined by your operational procedures, it is not necessary to perform a calibration. For example, let us use ±0.2 pH as your tolerances for pass/fail of a pH sensor reading in a calibration buffer. If the sensor reads within this value, in the offset (7 pH) and span buffers, (4 pH), the sensor needs no further action and can be reinstalled into the process. A calibration is not necessary. Repeat this exercise every few days until you see a change in either the level of debris/foulant on the electrode and reference surfaces, or more than the ±0.2 pH deviation as shown in the example nearby.
To a certain extent, the procedure nearby sets the benchmark for time between cleaning and calibration. Now, one needs to determine whether the sensor needs just a cleaning or a cleaning and re-calibration. This is done easily by first making sure the sensor is clean. (Refer to the section on Cleaning pH sensors). Cleaning may be as easy as rinsing the sensor in water or as complicated as using acid or caustic solutions to remove the contaminate buildup that has occurred.
See Figure 1: Due to manufacturing criteria, pH measurements are sometimes made in areas of the process plant where accessibility to the pH sensor is less than ideal.
About Cleaning Sensors
For the pH sensor to maintain an accurate reading of the process pH, the pH sensor must remain clean. Specifically, the glass measuring electrode cannot become coated and the reference electrode assembly must not become coated, plugged or otherwise contaminated by the process solution.
If the pH sensor has a slight coating or scaling, this might be removed using a water jet from a faucet or spray bottle.
More entrenched coatings may require the use of a gentle acid brush or tooth brush to carefully remove the coating.
Depending on the nature of the scale or coating, you may find it necessary to dip the sensor in a hot water solution containing Dawn® dishwashing detergent and then lightly scrub the electrode for a few seconds to facilitate cleaning.
For a sensor coating on which the detergent cleaning does not suffice, you may have to dip the brush in a 2% HCI acid solution and then lightly scrub the electrode for a few seconds to facilitate cleaning. Alternatively, you may have to allow the sensor (electrodes) to soak in a similar solution for a few minutes to really work at attacking the contaminant.
Figure 2: Quality buffers are traceable, can cover the full pH measurement range and are usually available in package sizes of 18 ml up to 5,000 ml.
Immediately after cleaning, rinse the sensor in water and allow the sensor to soak in tap water or a 7 pH buffer solution for a few minutes to allow the pH sensor to stabilize.
Table 1: Cleaning a pH sensor.

In general, the cleaning procedure is as follows:
1. Keep sensor as reasonably clean as possible.
2. Remove the bulk of contaminants by carefully blotting/wiping away debris. Be careful not to rub too vigorously as this may cause static charge.
3. Rinse the sensor in warm tap water or distilled water.
4. Prepare a cleaning solution containing a soap and water mixture. Use dishwashing detergent and warm water. Use only soaps that do not contain abrasives or lanolin.
5. Soak the sensor in this solution for up to five minutes and then gently or while soaking, use a soft bristle brush to gently scrub the bulb and reference area of the sensor.
6. Rinse the pH sensor in warm tap water and check/standardize the sensor in buffer solutions.
If the readings in buffers are still out of tolerance:
7. Soak the sensor in 5-10% HCI acid solution for a few – less than five – minutes.
CAUTION!
Do not use this procedure if the sensor has been used in a solution containing cyanide as this may produce poisonous cyanide gas.
8. Rinse the sensor in warm tap water and then place the sensor into a mild soap solution for a minute or two to neutralize any remaining acid and let the sensor come to equilibrium.
9. Rinse in warm tap water and check/standardize the sensor in buffer solutions.
Should the above procedures yield results that are within your operational tolerances, the pH sensor is once again suitable for use. However if the above procedures do not bring the readings of the pH sensor within tolerance, it is time to replace the sensor.
Author
Fred Kohlmann is a manager for analytical products with Endress+Hauser. Since 1976, he has been involved in engineering, design service, marketing, and sales of online analytical water quality and process control instrumentation. He has taught accredited pH courses and written publications, including “What Is pH and How Is It Measured?” Web: www.us.endress.com/pH.
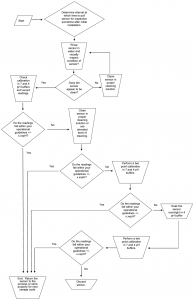
DECISION TREE
The decision tree is a quick graphic interpretation of the procedures outlined in the text.





Comments