March 2010 Vol. 237 No. 3
Features
Quality Assurance In Corrosion Prediction Of Multiphase Lines
One of the key factors in meeting the design life requirement of an oil and gas flow or export line depends on the accuracy of corrosion prediction and its management. Therefore, the assessment of internal corrosion of a pipeline system must also include examining the mechanism by which corrosion is expected and understanding how it is most likely to occur.
In many cases, corrosion mechanisms can produce localized conditions that may not reflect in the bulk of the produced fluids. Localized MIC corrosion – occurring in an export oil line through sediment containing micro organisms – is not usually accounted for by corrosion models in predicting corrosion rate. We have examined a few corrosion-prediction models in relation to flow and export line corrosion analysis and reviewed briefly the outcome in line with the Total Quality Management system (TQM) as applied in a typical project.
Here is a general review of corrosion mechanism and corrosion rate prediction. The complex nature of CO2-H2S corrosion makes accurate rate prediction difficult, and localized effects often hinder prediction of corrosion rates. Factors such as pressure, temperature, flow rate, flow profile, oil properties, pipe material, pipe routing, pH and a few other variables would influence the rate of corrosion predicted by a corrosion model in different pipe line sections. Flowing conditions often change along the route of a single pipeline and localized conditions can make some areas along the pipeline more likely to experience corrosion than other areas. Identification of these flow conditions and locations along the pipeline with high susceptibility to corrosion is one of the primary functions in devising an effective corrosion-management system.
Ideally, settling of solids and the presence of bacteria, or presence of elements such as sulphur which can affect formation of stable corrosion-product films, must be evaluated in conjunction with flowing conditions to determine the effect along each pipeline. Although some mechanistic and empirical corrosion models claim to have considered some such localized effects, they often do not stand up to the design integrity requirements that a project demands. An effective corrosion management system thus becomes an integral component of pipeline corrosion rate prediction and the design life. (1)
We will review some of the key factors affecting the corrosion rate that are common to most of the corrosion models commercially available before examining how effectively these factors have been adopted in the case of few models.
Effect Of Flow
The main ways in which flow may affect CO2 corrosion can be distinguished based on whether flow conditions are conducive to protective layer formation:
- In the case of corrosion where protective layers do not form (in the absence of inhibitors), the main role of the flow is to enhance transport of species toward and away from the metal surface. This may lead to the increase in corrosion rate.
- When the protective carbonate layers form (typically in higher pH produced water), or when inhibitor films are present on the steel surface, the above-mentioned effect of flow becomes insignificant.
Effect Of pH
Whether CO2 or H2S or combined corrosion mechanism, pH has a strong influence on the corrosion rate. The most important effect of pH is indirect and relates to how pH changes conditions for formation of ferrous carbonate layers in a CO2-driven corrosion process. High pH here results in a decreased solubility of ferrous carbonate and leads to an increased precipitation rate and a higher scaling tendency. Similar is happening with S2- i.e., the concentration also increases with increasing pH. On the other hand, the precipitation processes consume both CO3 and S2-. However, CO3 is rapidly replenished by the abundant amounts of dissolved CO2 while S2- is not replenished due to much smaller quantities of dissolved H2S in the solution so the S2- concentration plummets.
On balance, the local super saturation of FeCO3 at the metal surface increases with time due to the increase of both Fe2+ and CO3 concentration. However, the local super saturation of FeS decreases as any excess in S2- is rapidly consumed by the precipitation of FeS. Since the kinetics of both FeCO3and FeS precipitation depend strongly on the respective super saturation, FeCO3 will gradually prevail over FeS in the mixed scale when given enough time.
Effect Of Multiphase Flow
The simultaneous flow of oil and water in crude oil production and transportation pipelines is a common occurrence, from the well perforations to the final stages of separation. Corrosive gases such as carbon dioxide and hydrogen sulphide are also commonly present in these systems. Typically, at low water cuts this is not an issue as all the water is entrained by the flowing oil. As the water cut increases, water “breakout” may occur, leading to segregated flow of separate layers of water and oil phases. Therefore, the possibility of corrosion is high where the water phase wets the pipe wall (typically at the bottom).
Effect Of H2S Partial Pressure
Corrosion rate of mild steel at extremely low H2S controls the corrosion rate even when present in minute amounts, H2S reduces the pure CO2 (H2S?free) corrosion rate by 3-10 times due to formation of a thin mackinawite film. (2)
Effect Of CO2 Partial Pressure
In the case of scale?free CO2 corrosion, an increase of CO2 partial pressure, typically leads to an increase in the corrosion rate. With the increase of CO2 partial pressure the concentration of H2CO3 increases and accelerates the cathodic reaction, and ultimately the corrosion higher CO2 partial pressure leads to an increase in bicarbonate and carbonate ion concentration and a higher super-saturation, which accelerates precipitation and protective layer formation.
Effect Of Temperature
Temperature accelerates all the processes involved in corrosion: electrochemical, chemical, transport, etc. The corrosion rate steadily increases with temperature, and this is the case at low pH when precipitation of ferrous carbonate or other protective layers does not occur. The situation changes markedly when solubility of ferrous carbonate is exceeded, typically at a higher pH. In that case, increased temperature accelerates rapidly the kinetics of precipitation and protective layer formation, decreasing the corrosion rate.
Export Line Corrosion Characteristics
The oil entering the export pipeline has a specified basic sediment and water but is generally non-corrosive to steel. However, during the later stages of the production life, the flow rates in the pipeline will be low in that it could give rise to water dropout. If this occurs, the corrosion threat could be due to the microbiologically influenced corrosion (MIC). MIC is relatively difficult to predict and most corrosion models do not effectively consider this factor.
Total Quality Management Framework
It is important that corrosion-management activities related to the production facilities are carried out within a structured total quality management (TQM) framework incorporating the requirements of pipeline design, installation, production and export of oil and gas. Such a management program would have a direct or indirect effect on the overall life-cycle performance and the technical integrity. Quality assurance (QA) of two such corrosion management activities within the context of this article relate to (a) selection and execution of a corrosion model and (b) assessing integrity/flow assurance (production) behavior.
As for the QA, there are several approaches in developing corrosion prediction models: mechanistic, empirical and hybrid. However, the experience we have in the various software products is similar to what was already reported by Singh et al (3) leading to skewed or conservative predictions.
It is therefore prudent to assume greater safety margin levels by applying worst-case conditions in predicting design life and carrying out systematic and planned technical integrity audits including project specific software testing during the selection process. We have thus successfully designed flow and export pipelines in API grade carbon steel pipe material avoiding costly CRA (corrosion-resistant alloys) on TQM-based integrity assessment factors such as pipe length, time in operation, water cut, amongst other factors assists in quantifying life cycle with reasonable reliability. Details on such TQM-based corrosion management programs are outside the scope of this article.
Variation In Corrosion-Prediction Rate By Different Models
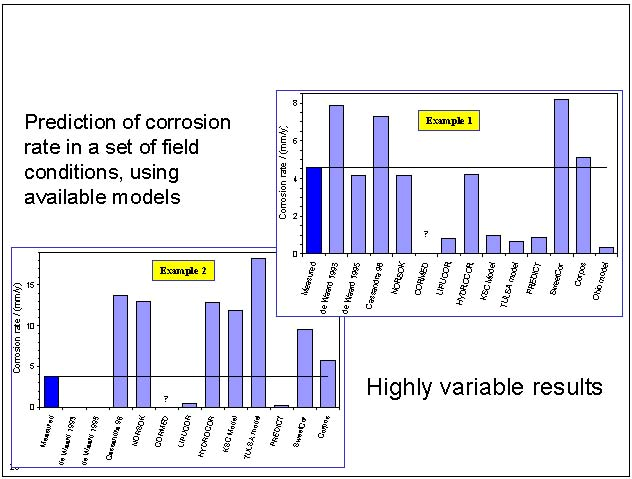
Corrosion-prediction rate as a function of protective film formation within an overall corrosion mechanism has been considered as a major contributing factor in corrosion rate calculation in some, but not all models. For example, the De Waard model makes a moderate account whereas Hydrocor assumes only minor effect of the film (4); the Freecorp model tends to consider this a major factor along with the oil-wetting effect, but the ECE-4 model assigns a moderate account for protective film in the corrosion rate calculation. Figure 1 by Gabetta (5) demonstrates significant variations in the corrosion rate.
Figure 1: Variation in corrosion prediction rate (5).
As a part of TQM we selected some of our calculated data and presented it in Table 1 to further examine this variation.
Accordingly we carried out some limited calculation using different models. See Table 2 which uses the calculated data basis from Table 1.
Discussion And Observation
The results show significant variation in the general corrosion rates from the three models presented in Tables 1 and 2 considering identical input. In case of ECE-4, it provides a measure for general corrosion as well as pitting. Both Freecorp and ECE-4 evaluate corrosion rates taking into account the conjoint effect of both CO2 as well as H2S, the two main corrosion species in the flow lines. Norsok is primarily a CO2-driven model6, accordingly, for low H2S, Norsok was included with Freecorp and ECE-4, thus all three models had been utilized within their capability ranges. All three models provide a general corrosion rate as a function of time that forms the basis of pipeline design life prediction. The ECE-4 that provides an account for isolated pittings adds a critical dimension to the prediction process of pipeline design life. However, pipeline design life calculation based only on the general corrosion rate in the presence of pittings could provide a misleading estimate. This is because unlike general corrosion that affects the pipeline integrity least, pitting corrosion results in a local loss of material in a much shorter operation period that can act as stress raiser, leading to a premature failure. To assure design integrity under the circumstance, timely prediction of location and type of corrosion using an alternative software (7) is essential. It then allows making an inspection and test plan based on the local corrosion alerts as a part of the TQM-based corrosion management program.
The understanding of CO2-H2S-based corrosion, as well as localized corrosion such as MIC, are vital in the selection of an effective corrosion model for flow and export lines.
Authors
Nimmo Dragomelo is employed by Petrofac International Ltd, Sharjah, UAE. He holds a Ph.D. degree in Engineering Science from Warwick University, UK. He is also a chartered metallurgist and a corporate member of the Institute of Materials, Minerals and Mining, UK, and a Fellow of the Institute of Quality Assurance, London, UK. He is an experienced quality manager with 25 years of experience in industries related to oil and gas, petrochemical, chemical, construction and nuclear in Western Europe and North America. Petrofac International is both an operator and EPC and he is a Senior Member of the Corporate Quality team. He can be reached at nimmo.dragomelo@petrofac.com.
Shahab Soltani, with Petrofac International Ltd, Sharjah, UAE, holds BSc and MSc degrees in Metallurgy. He is an experienced welding engineer in offshore and land-based oil and gas flow lines with a strong corrosion background. He has published articles related to pipe-weld quality management. He can be reached at Shahab.Soltani@petrofac.com.
References
1. Review of corrosion management for offshore oil and gas Processing/Capcis Limited for the Health and Safety Executive, UK.
2. Srdjan Neši?, Jiyong Cai and Kun-Lin John Lee, “A multiphase flow and internal corrosion prediction model for mild steel pipelines” Institute for Corrosion and Multiphase Technology/Ohio University, 342 W. State St, Athens, OH 45701; Paper/05556/Corrosion 2005.
3. Binder Singh, Kana Krishnathasan, Tawfik Ahmed “Predicting Pipeline Corrosion” Pipeline and Gas Technology, October 2008.
4. R. Nyborg, “Overview of CO2 Corrosion Models for Wells and Pipelines”, CORROSION/2002, Paper No. 02233, NACE International, Houston, 2002.
5. G. Gabetta, “Corrosion and fitness for Service” Eni San Donato Milanese, Italy.
6, NORSOK standard No. M-506 March 2005, CO2 Corrosion rate calculation model, www.standard.no/PageFiles/1178/M-506d1r2.pdf.
7. Western Corrosion Technologies: Corrosion Watch.





Comments