February 2013, Vol. 240 No. 2
Features
Hydrocarbon Dew Point Measurement Using Gas Chromatographs
The determination of hydrocarbon dew point (HCDP), the temperature at a defined pressure at which hydrocarbon liquids begin to form, has become critical for the natural gas industry. A big reason is that producers are focusing on extracting heavier gases in traditional and shale plays in an effort to produce more profitable natural gas liquids (NGLs) rather than the natural gas that is selling at historical lows. This has increased the risk of hydrocarbon liquids entering or forming in gas transmission networks if this rich gas is not processed fully.
Hydrocarbon liquids in the gas stream can cause hydrate formation, increase compression costs, cause issues with pressure regulator freezing, and lead to damage of gas turbines and other end-user equipment. Additionally, when the hydrocarbon liquids entering the transmission network are not measured through gas metering stations, or the high energy heavy hydrocarbons drop out as condensate (or “drip”) in the transmission network, the energy content of the gas leaving the network is less than the energy of the gas entering the network, resulting in increases in lost and unaccounted for (LAUF) energy. Therefore, it is understandable that custody transfer agreements are increasingly specifying limits for the hydrocarbon dew point.
The traditional method of determining the hydrocarbon dew point online is to use a chilled-mirror device that reduces the temperature of a mirror in a measurement chamber filled with the natural gas until enough hydrocarbon mist condenses on the mirror to be detected. Other dedicated HCDP analyzers using different measurement techniques are also available; however, they all provide a HCDP only at a single pressure and are dedicated analyzers that provide a single measurement.
An alternative to a dedicated HCDP analyzer is using an equation of state (EOS) to calculate the hydrocarbon dew point at any pressure from the composition obtained from a gas chromatograph (GC). By entering the composition of the natural gas into a recognized equation of state, the theoretical HCDP can be calculated for any pressure as well as the cricondentherm (the highest dew point temperature at any pressure). The validity of the calculated value depends on the accuracy of the composition used especially for the higher carbon number hydrocarbons (C6 to C9). The two most commonly used and accepted equations of state are:
1) Peng-Robinson (PR) (1976) equation of state (1): This is the most commonly used method for “pipeline quality gas.”
2) Redlich-Kwong-Soave (RKS) (1972) equation of state: An improvement by Soave on the original Redlich-Kwong (1949) equation of state.
Gas chromatographs are already used in custody transfer measurement to determine the energy content, compressibility, density, and other physical properties. Therefore, using a gas chromatograph to determine the HCDP using an EOS provides additional valuable information from already required equipment.
The ability to calculate the HCDP at any pressure opens up some very practical applications that can significantly improve measurement and lower lost and unaccounted for energy. Flow-meters used in custody transfer (such as orifice meters, turbines and ultrasonic flow-meters) are designed to measure single-phase gas flow with uncertainties typically below +/ 0.5%. However, the uncertainty significantly increases when hydrocarbon liquids form. Zanker and Brown (20002) reported errors up to five percent with stratified flow through ultrasonic meters. Some meters have the ability to detect when there is two-phase flow; however, this is too late to take corrective action as the flow metering accuracy is already compromised.
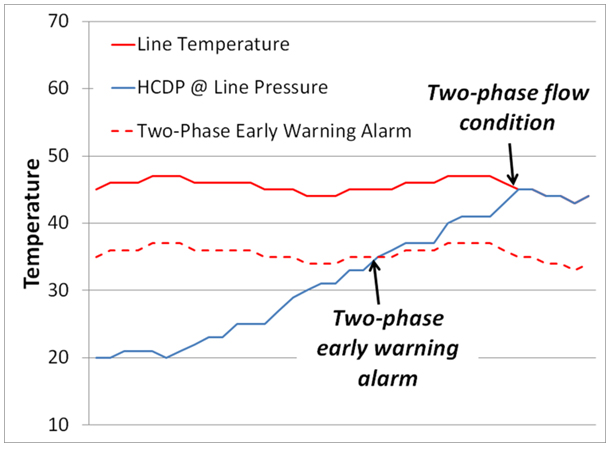
A gas chromatograph that is capable of calculating the HCDP at any pressure enables a very useful operational diagnostic that can be used to avoid two-phase flow, and therefore incorrect measurement. In normal single-phase gas flow conditions, the HCDP of the gas is less than the flowing temperature and all of the hydrocarbons are in the gas phase. If the gas becomes richer and the HCDP of the mixture increases above the pipeline temperature, the heavy hydrocarbons will drop-out into the liquid phase and the remaining gas will have a HCDP equal to the flowing temperature. By calculating the HCDP at the current pipeline pressure and comparing it to the current pipeline temperature, the operator can determine if there is two-phase flow. Even better, by setting an alarm so that if the HCDP temperature is within a certain range (say 10°F) of the flowing temperature, the operator can have an early warning that two-phase flow (and thus, inaccurate flow measurement) is about to occur (Figure 1). When this alarm occurs, the producer can make corrective actions in their process to avoid the HCDP increasing further and moving into the two-phase flow region where the accuracy of the flow measurement is degraded.
Figure 1: The chart shows how comparing the HCDP at pipeline conditions (calculated in a gas chromatograph) and the flowing temperature can be used to provide an early warning of two-phase flow.
The pipeline pressure and temperature is already measured as a part of the custody transfer measurement system, so the gas chromatograph can receive the pressure value from an analog input or via the Modbus interface to the supervisory system. The “two-phase early warning” alarm can be configured in the supervisory system and be incorporated into the station alarm management system.
Gas Chromatograph Requirements
The most common gas chromatograph configuration used for natural gas custody transfer is the “C6+” application. In this application the analyzer measures all of the hexane and larger components as a combined back-flushed component. The controller then calculates theoretical values for the hexanes, heptanes, and octanes used in the energy and physical properties calculations from fixed ratios of the measured C6+ concentration. The ratios are usually determined by an agreement between the custody transfer parties and are often one of the common industry “splits” shown in Table 1. While the effect of using these assumed mixtures of the C6+ fraction for calculating energy content, compressibility, density, and the other common physical properties is small and below other sources of uncertainties in the measurement system.

Table 1: Fixed ratios used to calculate theoretical C6/C7/C8 composition from back-flushed C6+ analysis values.
However, when condensation occurs in natural gas it is the heavier components that form the liquid phase. Therefore, the effect of the heavy hydrocarbons on the hydrocarbon dew point is disproportionate to the concentrations. Using fixed ratios of the C6+ fraction to calculate the hydrocarbon dew point will result in very large errors (Figure 2) and will not provide enough accuracy to be practical.
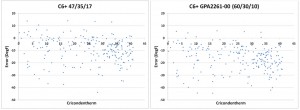
Figure 2: The plot of the error between the HCDP calculated with a C9+ analysis and calculated with the fixed ratios of a C6+ fraction shows significant errors that are impractical for meaningful use.
To overcome this limitation, the gas chromatograph must analyze the heavy hydrocarbons that will drop out into the liquid phase. Ideally, a gas chromatograph configured to do an extended analysis up to C12’s would provide the lowest uncertainty. However, this requires a flame-ionization detector that is often not practical for the remote, un-manned locations at which custody measurement often occurs.
To balance the accuracy needs of the HCDP application with the reliability and usability needs of the remote end-user, a C9+ application can be employed. This application uses two robust thermal-conductivity detectors (TCD) more suited to the typical custody transfer environment than the flame-ionization detector, and provides results within +/- 5°F of the values calculated using a full C12 analysis. The calculation can be characterized further to match isomer ratios determined by detailed spot laboratory analysis when the source of the gas is relatively constant.
The extended C9+ analysis is achieved by performing a parallel analysis. On one analytical path, the C1 to C5 components, nitrogen and carbon-dioxide are measured using the same analytical method as the common C6+ application with the back-flushed C6+ component ignored. On the second analytical path the heavier components are grouped into hexanes, heptanes and octanes, with the components larger than n-octane back-flushed to provide a C9+ grouped component value. In the snapshot shown in Figure 3, the C1 to C5, carbon-dioxide, and nitrogen analysis is shown with the blue trace, and the hexanes, heptanes, octanes and C9+ analysis is shown with the red trace.
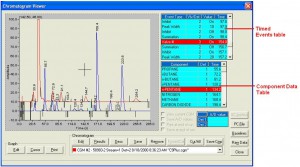
Figure 3: C9+ Chromatograms.

Figure 4: Rosemount Analytical 700XA Gas Chromatograph from Emerson Process Management.
The ability of the C9+ gas chromatograph to calculate the hydrocarbon dew point at any pressure, and thus determine the hydrocarbon dew point at the flowing conditions, provides pipeline operators the ability to take corrective action before heavy hydrocarbons enter their network. Maintaining single-phase flow through the flow meter and thus maintaining flow measurement accuracy can result in significant improvements in lost and unaccounted for gas, directly improving profitability. As this functionality is an extension of the capabilities of the gas chromatograph that is already needed for other functions in the custody transfer application and well understood by metering personnel, it provides an easy method for pipeline operators to protect their gas pipeline systems from hydrocarbon liquids without significant additional capital expenditure or engineering and operational effort.
Author:
Shane Hale has 15 years’ experience, starting as a field technician and working his way through project engineering and commissioning to his current role managing the gas chromatograph products at Emerson Process Management. He is a member of the AGA Transmission Gas Measurement Committee and is involved with the ISO standards working groups. Info: www.RosemountAnalytical.com.
Literature Cited
1) Peng, D.Y. and Robinson, D.B., A New Two-Constant Equation of State, Industrial and Engineering Chemistry: Fundamentals, Vol. 15 (1976), pp. 59-64.
2) Zanker, K. G., and Brown, G. J., The Performance Of A Multi-Path Ultrasonic Meter With Wet Gas. 18th North Sea Flow Msmt Workshop (2000).





Comments